Abstract
Introduction: Melphalan flufenamide (melflufen) is a peptide-drug conjugate with unique PK properties that rapidly penetrates cells, where it is metabolized to melphalan either directly or through an intermediate metabolite, desethyl-melflufen. Melflufen has only been administered via central venous catheter (CVC); however, peripheral venous catheter (PVC) administration may be preferred by patients if safety and tolerability are acceptable. The ongoing PORT study (NCT04412707) aims to compare the PK of melphalan after central and peripheral administration of melflufen, and to assess the local tolerability of peripheral administration of melflufen. The overall efficacy and safety of melflufen in patients with RRMM will also be evaluated.
Methods: Patients (following ≥2 lines of prior therapy) were randomized (1:1) to melflufen 40 mg (administered on Day 1 of each cycle; combined with weekly oral dexamethasone 40 mg [20 mg for patients aged ≥75 years] on Days 1, 8, 15, and 22) either via PVC in Cycle 1 then CVC in Cycle 2 (Arm A) or via CVC in Cycle 1 and then PVC in Cycle 2 (Arm B). From Cycle 2 (Arm A) or Cycle 3 (Arm B) onward, patients received melflufen via CVC. PK sampling was performed frequently during and after the 30-minute melflufen infusion. Primary endpoints were maximum observed concentration (C max), area under the concentration-time profile from start of infusion to both last measurable concentration (AUC 0-t) and infinity (AUC 0-inf) for melphalan, and frequency and severity of PVC-related local infusion-site reactions. Secondary endpoints included PK variables for melflufen and desethyl-melflufen and general safety and tolerability. PK parameters after CVC and PVC administration were compared using bioequivalence methods. Patient satisfaction and nurse convenience after CVC and PVC administration (Day 1 of Cycles 1 and 2) were also explored.
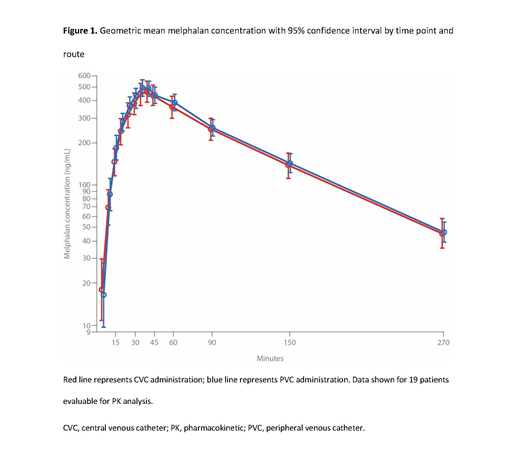
Results: At data cutoff (June 2, 2021), 27 patients had received melflufen (median age 67 years; 48.1% male), of whom 19 patients received at least 2 doses and were evaluable for PK analysis. Melphalan C max, AUC 0-t, and AUC 0-inf met bioequivalence criteria for CVC and PVC administration, as demonstrated by a 90% confidence interval (CI) for the ratio of means within 80-125%. Geometric mean melphalan concentration over time by CVC vs PVC is shown in Figure 1. For melflufen, the ratio of means was 107-117% for the PK parameters, with all upper 90% CIs above 125%. For desethyl-melflufen, AUC 0-t and AUC 0-inf met bioequivalence criteria and the 90% CI for C max was marginally above the upper limit (127%). Melflufen disappeared rapidly from plasma after the end of infusion, with an average half-life of 5-7 minutes. Melphalan C max was observed on average 7-9 minutes after the end of melflufen infusion for both routes of administration, which reflects the delay in distribution of melphalan from tissues to plasma. Furthermore, there were no local or systemic tolerability issues reported, and patient-reported satisfaction and nurse-reported convenience were comparable for CVC and PVC melflufen administration. The overall melflufen safety profile was in line with previous studies.
Conclusions: Systemic melphalan exposure is similar after melflufen PVC and CVC administration. Differences between PVC- and CVC-related PK parameters for melflufen and desethyl-melflufen are considered to have no clinical consequences because their plasma-exposure duration is short. There were no local reactions after melflufen PVC administration.
Minarik: Amgen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. Micheva: Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Andzhelini Farma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Usenko: AbbVie: Consultancy, Honoraria, Other: Clinical Trials Investigator; Janssen: Consultancy, Honoraria, Other: Clinical Trials Investigator; Pfizer: Consultancy, Honoraria; Acerta: Other: Clinical Trials Investigator; Ascentage: Other: Clinical Trials Investigator; Celgene: Other: Clinical Trials Investigator; Il-Yang: Other: Clinical Trials Investigator; Karyopharm: Other: Clinical Trials Investigator; Oncopeptides: Other: Clinical Trials Investigator; Rigel: Other: Clinical Trials Investigator; Takeda: Other: Clinical Trials Investigator; UCB: Other: Clinical Trials Investigator. Mikala: Takeda: Honoraria; Celgene: Honoraria; Amgen: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Jerling: Oncopeptides: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Xie: Oncopeptides: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.